GREEN DYE MASTER MIX/PROBE QPCR MASTER MIX
ORDERING INFORMATION
2x GREEN DYE MASTER MIX (HOT START)
| Catalog # | Number of 50 µl Reactions |
Volume | ROX (5 µM) |
Flourescein (500 nM) |
Price (EUR) |
| # S 50 |
50 |
1.25 ml | 250 µl |
100 µl |
59,50 |
| # S 200 | 200 |
4 x 1.25 ml | 1000 µl | 400 µl |
185,50 |
| # S 400 | 400 |
8 x 1.25 ml | 2000 µl | 800 µl |
352,00 |
| # S 2000 | 2000 |
50 ml |
10 000 µl | 4000 µl |
1520,00 |
Please add on your order the type of reference dye needed: ROX or Fluorescein.
2x PROBE QPCR MASTER MIX (HOT START)
| Catalog # | Number of 50 µl Reactions | Volume | ROX (5 µM) |
Flourescein (500 nM) |
Price (EUR) |
| # P 50 |
50 |
1.25 ml | 250 µl |
100 µl |
51,50 |
| # P 200 | 200 |
4 x 1.25 ml | 1000 µl | 400 µl |
161,50 |
| # P 400 | 400 |
8 x 1.25 ml | 2000 µl | 800 µl |
306,00 |
| # P 2000 | 2000 |
50 ml |
10 000 µl | 4000 µl |
1322,00 |
Please add on your order the type of reference dye needed: ROX or Fluorescein.
DESCRIPTION
The 2x Green DYE Master Mix and the 2x Probe qPCR Master Mix contain all reagents required for Real Time PCR and are designed to make PCR as easy and simple as possible. All components (including Hot Start Taq DNA polymerase) are provided in an optimized concentration. With 2x Green DYE Master Mix all you need to do is to add primers and template DNA, with the 2x Probe qPCR Master Mix addionally probes, thus minimizing the pipetting effort and possible sources of error. ROX or Florescein as reference dye is included in a separate vial.
The Green DYE allows the universal detection of every PCR product. The Green DYE Master Mix and the Probe qPCR Master Mix can be successfully used with every commercial available Real Time PCR instrument.
The highly specific and sensitive Hot Start Polymerase and the extensively purified dNTP´s lead to excellent results of PCR efficiency, correlation coefficient and slope.
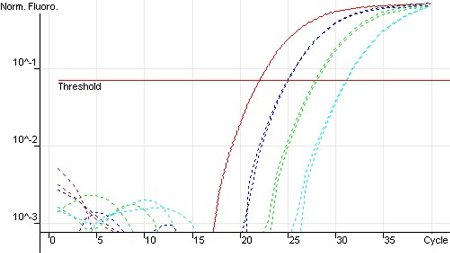
Efficiency of the amplification of human GAPDH using log fold serial dilutions from 1 ng to 1 pg on the RG-3000 (Corbett Research).
MELTING CURVE
For Green DYE based amplicon detection, it is important to run a dissociation curve following the real time PCR. This is due to the fact that Green DYE will detect any double stranded DNA including primer dimers, contaminating DNA, and PCR product from misannealed primer.
The Tm of the amplicon correlates with the maximum of the melting curve profile.
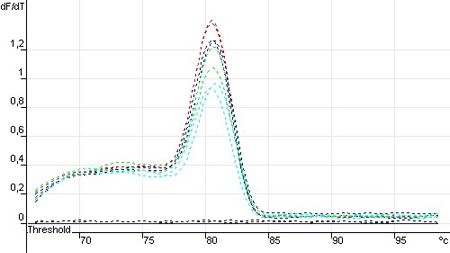
Derivative Melting Curve for Standard Curve Samples in Real Time, GAPDH Endogeneous Control
It is apparent that the maximum (melting temperature of the amplicon) occurs at 81.5°C. Also we can see that no contaminating products are present in this reaction. Contaminating DNA or primer dimers would show up as an additional peak separate from the desired amplicon peak
BASIC PROTOCOL FOR 2x GREEN DYE MASTER MIX
|
Component |
Volume (in µl) |
Final concentration |
|
2x Master Mix |
25 |
1x |
|
Primer mixture |
15 |
300 nM* |
| Template DNA |
10 |
100 – 300 nM |
* The concentration of primer in the mixture should not exceed 300 nM. Higher concentrations result in unspecific amplification and do not lead to more product. Recommended melting temperature of primers is 53 – 60°C.
BASIC PROTOCOL FOR 2x PROBE QPCR MASTER MIX
|
Component |
Volume (in µl) |
Final concentration |
|
2x Master Mix |
25 |
1x |
|
Primer mixture |
variable |
300 nM |
|
Probes |
variable |
200 nM |
|
Template DNA |
variable |
1 – 10 ng/ 50 µl |
|
Distilled,
sterile water |
Add to a final volume of 50 |
|
Recommended melting temperature of primers is 53 – 60°C.
CYCLING PROGRAM FOR 2x GREEN DYE MASTER MIX
|
Step |
Temperature (in °C) |
Time |
Cycles |
|
Initial
activation |
95 |
15 min |
1 |
|
Denaturation |
94 |
15 sec |
45 |
|
Annealing |
60 |
30 sec |
45 |
|
Extension |
72 |
30 sec |
45 |
CYCLING PROGRAM FOR 2x PROBE QPCR MASTER MIX
|
Step |
Temperature (in °C) |
Time |
Cycles |
|
Initial
activation |
95 |
10
min |
1 |
|
Denaturation |
95 |
15
sec |
50 |
|
Annealing |
60 |
30
sec |
50 |
|
Extension |
72 |
30
sec |
50 |
QUALITY CONTROL ASSAYS
|
FUNCTIONAL ASSAYS |
SPECIFICATION |
|
Quantitative PCR |
|
|
Slope |
-3.0 > slope > -3.6 |
|
Correlation Coefficient |
> 0.990 |
|
Efficiency |
110% > efficiency > 90% |
|
PHYSICAL ASSAYS |
SPECIFICATION |
|
DNA Assay |
no contamination |
|
Endonuclease Assay |
no activity |
|
DNase Assay |
no activity |
|
RNase Assay |
no activity |
TROUBLE SHOOTING
|
Observation |
Check |
|
Preparing the reaction mix |
|
|
Preventing contamination |
|
|
Poor
correlation |
|
|
Low quality
disposable material |
|
|
Preparing a plate |
|
STORAGE
Store at -20°C for 12 months. Multiple freeze-thaw-cycles should be avoided by preparing aliquots.

